In highly regulated industries like food, beverage, and pharmaceutical production, maintaining quality and consistency is non-negotiable. One of the foundational frameworks supporting these goals is Good Manufacturing Practices, more commonly referred to as GMP. Whether you’re designing a new production facility or upgrading existing lines, understanding GMP is essential for ensuring compliance and building trust with your customers.
What Are Good Manufacturing Practices (GMP)?
GMP refers to a system of guidelines that ensure products are consistently produced and controlled according to quality standards. These practices are established by regulatory bodies like the U.S. Food and Drug Administration (FDA) and are designed to minimize risks involved in any production process that cannot be eliminated through final product testing alone. GMP covers all aspects of production, from raw materials and equipment to facility cleanliness and employee hygiene.
Why GMP Matters in Sanitary Production
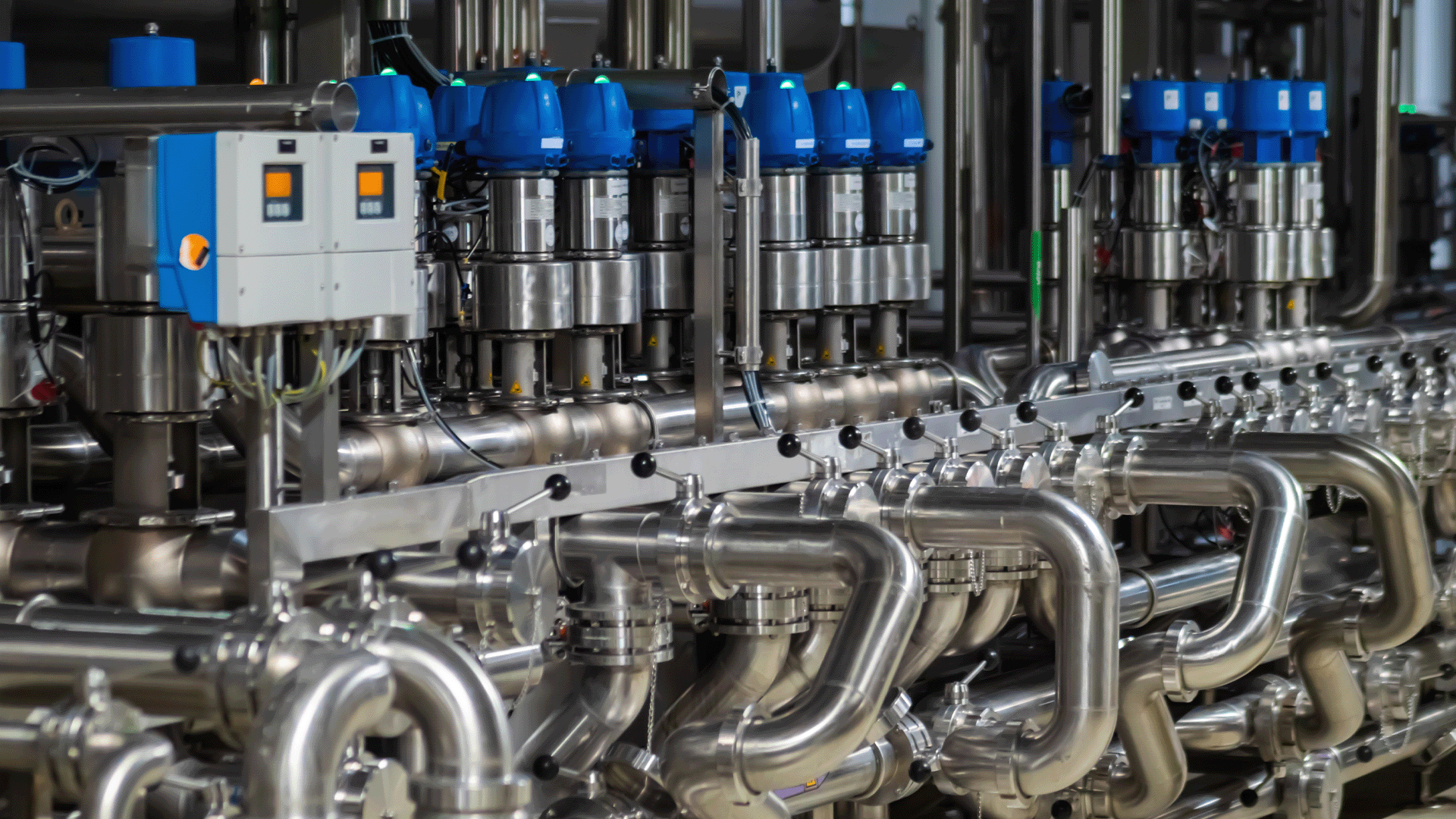
For manufacturers in the sanitary sector, GMP compliance is more than a regulatory requirement, it’s a strategic advantage. Choosing the right sanitary fittings, valves, gaskets, and tubing plays a vital role in supporting a GMP-compliant environment. Poor-quality components can compromise cleanability, introduce contamination risks, or result in process failures that jeopardize product safety.
Engineers and procurement professionals alike must ensure that every part of the production system is built with hygiene, traceability, and durability in mind. By investing in sanitary-grade components designed for Clean In Place (CIP) and SIP (Steam-in-Place) processes, you’re not only complying with GMP, you’re protecting your brand and your bottom line.
Core Components of GMP
While the specific details of GMP can vary depending on industry and region, several foundational elements remain consistent across the board:
- Personnel Hygiene: Ensuring that all employees are properly trained and follow strict hygiene protocols.
- Facility Design & Maintenance: Production areas must be cleanable, well-organized, and built to prevent cross-contamination.
- Sanitary Equipment: All equipment, including piping, fittings, and valves, must be designed for easy cleaning and maintenance.
- Process Documentation: Manufacturers must keep detailed records of processes, changes, and quality checks to ensure traceability.
- Quality Control: Ongoing inspection and testing ensure that products meet specifications and are free from contaminants.
- Material Handling: Raw materials, ingredients, and packaging must be stored and handled in a way that maintains integrity and prevents contamination.
GMP Starts With the Right Sanitary Components
GMP compliance begins at the component level. Selecting high-quality, 3-A certified sanitary parts that are easy to clean, corrosion-resistant, and designed for hygienic performance is essential for maintaining operational integrity. At Sanitary Fittings, we offer a full range of components engineered to support GMP adherence, helping our customers stay ahead of industry standards and inspections.
Whether you’re building a new system or auditing an existing one, we’re here to help ensure your facility meets the highest standards of cleanliness and safety. Explore our full range of sanitary fittings to support your GMP-compliant processes.